What is ozone
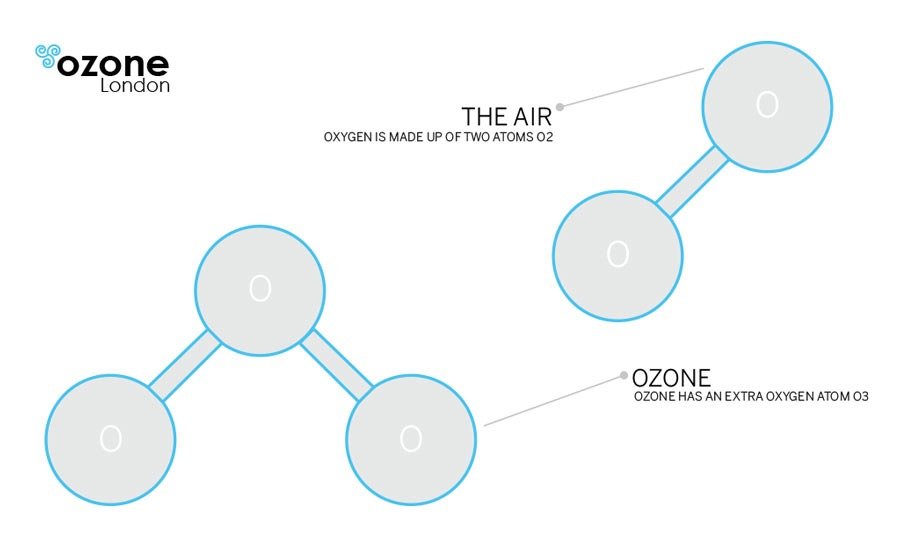
What is Ozone?
Ozone (O3) is colourless gas that has an odour most often described as the smell of air after a spring thunderstorm. O3 is an extremely constable gas and it is the strongest oxidant of the common oxidising agents. It is manufactured by passing air through the electrodes with high, alternating potential difference. It is formed by a high-energy input splitting the O2 (oxygen) molecule. Single ‘O’ rapidly combines with available O2 to form the very reactive O3.
O3 is produced in a natural environment, its function is to safe oxidation substances hazardous to health. Ozone treatment is an organic method, which does not leave any side effects, nor cause allergic reactions – properly done is harmless to humans and the environment. The treatment that is repeated at least twice a year improves the quality of life of allergy sufferers and asthmatics, as kills irritant fungi, mould and allergens.
Here is a lovely video from The Power of Ozone:
How Ozone inactivate viruses?
Typically, viruses are small, independent particles, built of crystals and macromolecules. Unlike bacteria, they multiply only within the host cell. Ozone destroys viruses by diffusing through the protein coat into the nucleic acid core, resulting in damage of the viral RNA. At higher concentrations, O3 destroys the capsid or exterior protein shell by oxidation.
Numerous families of viruses including poliovirus I and 2, human Rotavruses, Norwalk virus, Parvoviruses, and Hepatitis A, B and non-A non-B are among many others that are susceptible to the virucidal actions of ozone.
Most research on it’s virucidal effects have centred upon ozone’s ability to break apart lipid molecules at sites of multiple bond configuration. Indeed, once the lipid envelope of the virus is fragmented, its DNA or RNA core cannot survive.
In this study, we found that the effect of O3 on Poliovirus infectivity was both time- and dose-dependent, and PV1 could be completely eradicated after ozone disinfection for 105 s. It has been previously reported that PV1 infectivity is completely inhibited upon treatment with 0.2 mg/L ClO2 for 12 min, 0.4 mg/L ClO2 for 8 min, or 0.8 mg/L ClO2 for 4 min. This proves that ozone is more effective as a disinfectant than ClO2 (Chlorine dioxide).
Ozone is also effective as an air treatment in the hospitals and it has been proved to eliminate Phage and eukaryotic virus (murine norovirus MNV-1). “These findings suggest that ozone used at a low concentration is a powerful disinfectant for airborne viruses when combined with a high RH. Air treatment could therefore be implemented inside hospital rooms ventilated naturally.”
Ozone gas has been proven to kill the SARS coronavirus and since the structure of the new 2019-nCoV coronavirus is almost identical to that of the SARS coronavirus, it is relatively safe to say that it will also work on the new coronavirus though it must be noted that there is no studies to date except one that is current ongoing in China at the Institute of Virology In Hubei. Progress of that study has shown that it works and the study should be concluded soon and officially published in the journal Virology.
There are more than 17 scientific studies that show O3 gas is able to destroy the SARS coronavirus.
Ozone solution disinfectant at different concentrations was tested for inactivation of Severe Acute Respiratory Syndrome (SARS) virus. The results showed that the high concentration of 27.73 mg/ L ozone could inactivate SARS virus in 4 min.
Effectiveness of ozone against algal toxins
“Ozonation is an effective process for destruction of both intracellular and extracellular algal toxins. Essentially complete destruction of microcystins, nodularin and anatoxin-a can be achieved if the ozone demand of the water is satisfied.” (Yoo et al., 1995b; Chorus & Bartram 1999).
Drinking water disinfection
This is an interesting study about the drinking water disinfection. Among many “Ozone was the most efficient disinfectant for all types of microorganisms.” The estimated risks to health from disinfectants and their by-products are extremely small in comparison to the real risks associated with inadequate disinfection and it is important that disinfection should not be compromised in attempting to control such by-products. The destruction of microbial pathogens through the use of disinfectants is essential for the protection of public health.
In ‘Water and air ozone treatment as an alternative sanitizing technology’ study we read: “treatment is considered a safe and effective disinfectant tool for the decontamination of water and equipment [18] and even for food applications [20]; indeed, food safety is a top priority [21]. It may therefore be regarded as a valid alternate means of disinfection. (…) Among the gaseous sanitizers investigated in recent years, such as ozone, chlorine dioxide (ClO2), and cold plasma, ozone has proved the most effective [13], being a powerful oxidant for water treatment”.
To improve the quality of water “the concentration of ozone needed is very low. O3 has strong oxidizing properties. The speed of disinfection is very fast, and the inactivation effect is better than that of disinfectants such as chlorine, chloramines, and chlorine dioxide; it can also oxidize and degrade organic substances in water. The remaining O3 in water can naturally decompose and the quality of water can be improved”. (Inactivation of Poliovirus by Ozone and the Impact of Ozone on the Viral Genome)
Please make sure you watch this video of David Hart on how O3 works.
